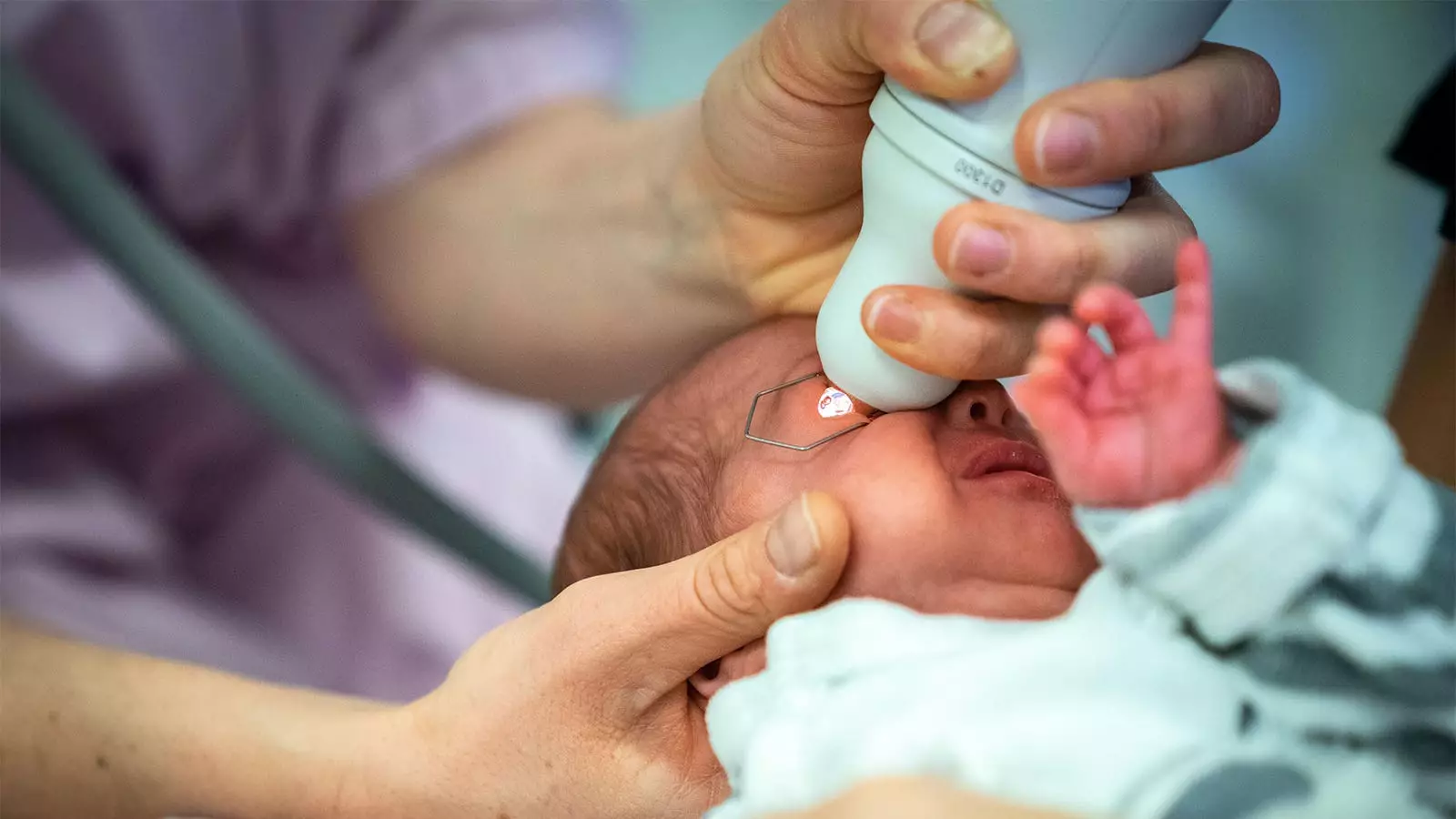
Retinopathy of prematurity (ROP) is a significant concern for the healthcare community, particularly in neonatal intensive care units (NICUs). As one of the leading causes of vision loss in children, ROP requires careful and frequent monitoring of preterm infants to facilitate early detection and timely intervention. This delicate balancing act of safeguarding visual health while minimizing the risk of systemic side effects forms the crux of an ongoing debate regarding the administration methods of mydriatic agents during screening procedures.
A recent randomized trial spearheaded by Dr. Asimina Mataftsi and her team at Aristotle University of Thessaloniki sheds new light on an innovative approach to ROP screening. Their comparative study evaluated the effectiveness and safety profiles of mydriatic microdrops versus standard drops in a cohort of 83 preterm infants. The findings revealed that microdrops not only maintained noninferior dilation at crucial time points but surpassed standard drops in Mydriatic efficacy, specifically at the 45-minute mark.
At the 45-minute interval, the microdrops demonstrated a mean difference of 0.12 mm in pupil dilation compared to their standard counterparts, with statistical significance endorsed by a Bonferroni-corrected 95% confidence interval (CI 0.01-0.23). Importantly, at 90 and 120 minutes, microdrops maintained noninferiority, suggesting a favorable profile for prolonged eye dilation without compromising effectiveness.
Adverse Effects and Clinical Implications
The effort to ensure safety during ROP screenings cannot be overstated; preterm infants are particularly susceptible to adverse effects resulting from systemic absorption of medications. Standard mydriatic agents have been linked to various cardiorespiratory and gastrointestinal complications, raising concerns among healthcare providers. The study found that standard drops not only produced lower oxygen saturation levels but also correlated with a higher percentage of hypertensive episodes in the monitored infants.
Critically, the median incidence of hypertensive episodes was lower in the microdrops group (0.10% vs. 0.14%, P=0.01), reinforcing the argument that microdrops may mitigate risks, offering a more favorable risk-benefit ratio, especially for this vulnerable population. The risk of cardiovascular and gastrointestinal adverse effects from conventional drops can result in significant challenges for NICU staff, who frequently report negative outcomes post-procedure.
In the MyMiROPS study, eligible infants were those with a gestational age of less than 32 weeks and/or a birth weight under 1,501 g, or those referred by an attending neonatologist due to associated comorbidities. The population included a mean gestational age of 29.7 weeks and an average birth weight of 1,277 g, with a gender distribution of approximately half being male.
The trial involved the random administration of three doses of either microdrops (6.5 µL) or standard drops (28 to 34 µL) of a diluted mixture of phenylephrine and tropicamide. Importantly, the researchers set a predefined noninferiority margin of -0.4 mm for pupil diameter measurement at specific intervals, a meticulous approach that reinforces the rigor of the study.
Despite the promising results, the researchers highlighted certain limitations, such as the lack of comprehensive safety outcome data for 30% of studied subjects. This gap points to the need for further research to confirm their findings across varied population demographics, dosage regimens, and ROP screening procedures.
The conclusion drawn by Mataftsi et al. underscores the imperative nature of adopting microdrops for this delicate patient group. They posit that the systemic absorption rate of microdrops can match that of conventional drops, while simultaneously minimizing potential systemic risks—an urgent step forward in pediatric ophthalmology.
The advent of mydriatic microdrops may herald a transformative era in ROP screening, marrying efficacy with enhanced safety in preterm infants. As healthcare professionals continue to refine methodologies to afford the best possible care in NICUs, this study stands as a testament to innovation that may reshape current clinical practices. Future endeavors in this realm promise to foster not only safer procedures but also improved health outcomes for one of the most vulnerable populations in medicine.